April 01, 2022 14:02
April 01, 2023 4:57
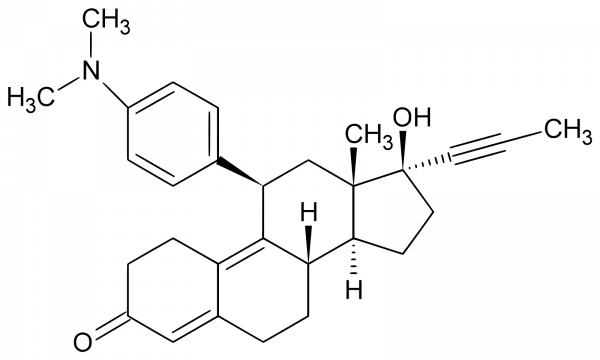
Mifepristone
Active substance
Mifepristone. Buy Mifepristone
ATC Codes
G03XB01 — Mifepristone
- G03XB — Progesterone receptor modulators
- G03X — Other sex hormones and modulators of the genital system
- G03 — Sex hormones and modulators of the genital system
- G — Genito urinary system and sex hormones
G03XB51 — Mifepristone, combinations
- G03XB — Progesterone receptor modulators
- G03X — Other sex hormones and modulators of the genital system
- G03 — Sex hormones and modulators of the genital system
- G — Genito urinary system and sex hormones
Chemical identifiers
UNII: 320T6RNW1F
CAS number: 84371-65-3
InChI Key: VKHAHZOOUSRJNA-GCNJZUOMSA-N
InChI: InChI =1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1
IUPAC Name: (1S,3aS,3bS,10R,11aS)-10-[4-(dimethylamino)phenyl]-1-hydroxy-11a-methyl-1-(prop-1-yn-1-yl)-1H,2H,3H,3aH,3bH,4H,5H,7H,8H,9H,10H,11H,11aH-cyclopenta[a]phenanthren-7-one
SMILES: [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@H] C1=CC=C(C=C1)N(C)C)C1=C3CCC(=O)C=C3CC[C@@]21[H]
Pharmacological group
Abortive (antiestrogenic) drugs.
Mechanism of action
The steroid drug has a weak androgenic effect and suppresses the development of progesterone by blocking progestogenic receptors. In addition, the drug enhances the contractility of the middle layer of the uterus (myometrium), activates the synthesis and release of a potent anti-inflammatory cytokine (interleukin 8), and increases the sensitivity of the muscle layer to prostaglandins. As a result, there is a rejection of the decidual (altered mucous membrane of the uterus, formed after embryo implantation) and the removal of the embryo. In addition, the substance inhibits ovulation, changes the endometrium, and blocks implantation of the zygote.
Pharmacokinetic properties
Absorption of the drug at a dosage of 200 mg, when taken per os (inside), occurs quickly. The maximum concentration of the substance in the blood is observed on average 1.3 hours after administration. The duration of the half-life is 18 hours. The drug is 98% bound to plasma albumins and glycoproteins. Oxidative biotransformation of Mifepristone is carried out in the liver. The medicine is excreted mainly with feces (90%) also 10% with urine.
Indications for use
- Termination of physiological pregnancy in the early stages, namely up to 77 days of amenorrhea;
- Artificial labor excitation for subsequent delivery during full-term pregnancy;
- Conservative preparation before surgical termination of pregnancy in the 1st trimester to soften and open the cervix;
- Artificial labor arousal in intrauterine fetal death in cases of contraindications to the use of oxytocin and prostaglandin;
- Monoclonal benign tumor of the uterus (leiomyoma), the size of which is no more than 12 weeks.
Abortion Pill Kit
Buy Abortion Pill Kit online in the US, Europe, and Worldwide
150$
Add to cart
AntiPREG Kit
Buy Abortion Pills online in the US, Europe, Worldwide
150$
Add to cart
Mifepristone Misoprostol combipack
Buy Mifepristone Misoprostol combipack online in the US, Europe, Worldwide
150$
Add to cart
Cytolog
Buy Cytolog online in the US, Europe, and Worldwide
150$
Add to cart
Cytotec
Buy Cytotec online in the US, Europe, and Worldwide
150$
Add to cart
Generic RU-486
Buy Generic RU-486 online in the US, Europe, Worldwide
150$
Add to cart
Mifeprex
Buy Mifeprex online in the US, Europe, and Worldwide
150$
Add to cart
Mifepristone
Buy Mifepristone online in the US, Europe, and Worldwide
150$
Add to cart
Misoprostol
Buy Misoprostol online in the US, Europe, and Worldwide
150$
Add to cart
Misoprost-200
Buy Misoprost-200 online in the US, Europe, Worldwide
150$
Add to cart
CONTRAKIT
Buy MTP Kit online in the US, Europe, Worldwide
150$
Add to cart
KillPreg Kit
Buy MTP kit online in the US, Europe, Worldwide
150$
Add to cart
Mifepristone + Misoprostol Kit
Buy Mifepristone + Misoprostol Kit online in the US, Europe, and Worldwide
150$
Add to cart
MTP Kit
Buy MTP Kit online in the US, Europe, Worldwide
150$
Add to cartContraindications to use
- Pregnancy for more than 77 days;
- Pathological ectopic fertilization;
- Unconfirmed pregnancy.
Common contraindications include:
- Hereditary porphyrin disease;
- Severe bronchial asthma;
- Chronic liver or adrenal insufficiency;
- Individual intolerance to hormonal drugs;
- Severe anemia.
In the presence of inflammatory diseases, eclampsia, severe gestosis, incorrect positions, or large fetal sizes, Mifepristone as an inducer of labor is contraindicated.
Side effects
Frequent side effects may be dyspeptic disorders (vomiting, nausea), stool disorders, infectious diseases after artificial abortions, uncontrolled contractions, or spasms of the myometrium for several hours. Less common complications include headaches, dizziness, fever, chills, decreased blood pressure, Quincke’s edema, allergic skin rashes, and uterine bleeding. In addition, cases of the lethal shock of an infectious-toxic nature and uterine ruptures during labor induction were recorded.
Since clinical studies are conducted under different conditions, the frequency of adverse reactions observed in one clinical examination cannot be directly compared with the frequency observed in another study. Therefore, it may not reflect the frequency of adverse reactions observed in practice.
The available information on commonly reported adverse reactions is based on data from studies conducted in the United States. In three clinical trials conducted in the USA, with the participation of 1,248 women up to 70 days pregnant who took Mifepristone at a dose of 200 mg orally, and after 24-48 hours — Misoprostol at a dose of 800 mcg buccal, women reported adverse reactions in diaries and during a survey during a control visit. These studies involved generally healthy women of reproductive age without contraindications to using Mifepristone or Misoprostol.
The pregnancy period was determined before the start of participation in the studies by the date of the woman’s last menstruation, clinical examination, and/or ultrasound results.
About 85% of patients reported at least one adverse reaction after taking Mifepristone and Misoprostol, but it was expected that many of them would say more than one adverse reaction. The most common adverse reactions (>15%) were nausea, weakness, intermittent fever (fever/chills), vomiting, headache, diarrhea, and dizziness.
The frequency of adverse reactions varies from study to study and may depend on many factors, including the patient population and the duration of pregnancy. Abdominal pain (abdominal pain) is expected in all patients undergoing the procedure of medical abortion/spasms, and information about their occurrence is not provided in clinical studies.
Mifepristone and Misoprostol aim to induce uterine bleeding and spasms that cause the termination of intrauterine pregnancy. Therefore uterine bleeding and spasms are the expected consequences of the action of Mifepristone and Misoprostol used in this procedure. Most women are expected to bleed more heavily than during heavy menstruation.
Infections and infestations: postabortem infections (including endometritis, endomyometritis, parametritis, pelvic organ infection, pelvic inflammatory diseases, and salpingitis).
From the blood and lymphatic system: anemia.
From the immune system: allergic reactions (including anaphylaxis, angioedema, urticaria, rash, itching).
From the side of the psyche: anxiety.
From the heart: tachycardia (including rapid pulse, palpitations, palpitations).
From the side of the vessels: fainting, pre-fainting (sudden weakness), loss of consciousness, hypotension (including orthostatic), dizziness.
From the respiratory organs, chest, and mediastinum: shortness of breath.
From the gastrointestinal tract: dyspepsia.
From the musculoskeletal system, connective and bone tissue: back pain, leg pain.
From the reproductive system and mammary glands: rupture of the uterus, rupture of ectopic pregnancy, hematometra, leukorrhea.
General disorders and disorders at the injection site: pain.
Features of interaction with other drugs
Specific studies of the interaction of Mifepristone with other drugs have not been performed. But it is known that the antibiotic Rifampicin, the hormonal drug Dexamethasone, and some anticonvulsants (Carbamazepine and Phenobarbital) can reduce the content of Mifepristone in the blood serum. Antifungal drugs such as Itraconazole and Ketoconazole and the antibiotic Erythromycin, on the contrary, increase the concentration of the drug in the blood.
External links
- Human Metabolome Database HMDB0014972;
- KEGG Drug D00585;
- KEGG Compound C07652;
- PubChem Compound 55245;
- PubChem Substance 46505795;
- ChemSpider 49889;
- BindingDB 50072024;
- RxNav 6964;
- ChEBI 50692;
- ChEMBL CHEMBL1276308;
- ZINC ZINC000003831128;
- Therapeutic Targets Database DAP000090;
- PharmGKB PA450500;
- Guide to Pharmacology GTP Drug Page;
- PDBe Ligand 486;
- RxList RxList Drug Page;
- Drugs.com Drugs.com Drug Page;
- Wikipedia Mifepristone;

 Français
Français